Telomeres as a guideline for appropriate treatment in slowing down and preventing the shortening of telomeres effectively, such as changing sleep behavior, exercising, managing stress levels, and consuming various nutrients.
Telomeres are the protective caps at the ends of chromosomes that prevent the loss of genetic information during cell division. The structure of chromosomes was discovered by Hermann Muller and Barbara McClintock through their studies on fruit flies and corn, respectively. They found that a special structure at the end of chromosomes serves to protect the tips of chromosomes, preventing them from being recognized as damaged DNA, preventing abnormal rejoining, or degradation of chromosome ends. In 2009, Elizabeth Blackburn, Carol Greider, and Jack Szostak were awarded the Nobel Prize in Physiology or Medicine for their discovery of how chromosomes are protected by telomeres and the enzyme telomerase, leading to extensive research in this field.
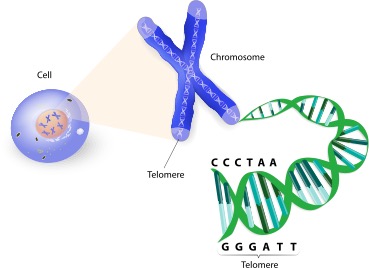
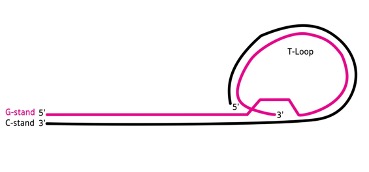
The structure of telomeres consists of non-coding base sequences that repeat in sets, such as “TTAGGG,” which is the base sequence found at the ends of chromosomes in vertebrates, including humans. The length of telomeres can range from several hundred to several thousand repeats. The single-stranded G-rich strand, also known as the 3′ end, extends outwards and folds back to form a large loop called the Telomere loop or T-loop. This structure resembles a paperclip and is stabilized by a group of proteins that specifically bind to the TTAGGG sequence of telomeres, maintaining the stability of the telomere ends.
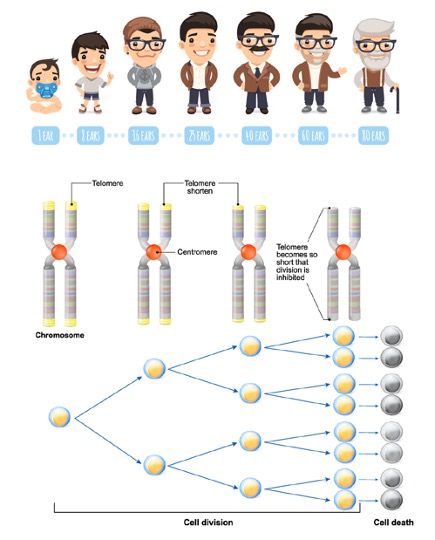
Due to the limitation in DNA replication, which results in the loss of the ends of DNA strands in each replication cycle, known as the “End Replication Problem,” as explained by Olovnikov, the working mechanism of the DNA polymerase enzyme synthesizes DNA strands like a train running on tracks, with the train representing the enzyme and the tracks representing the DNA strand. However, the train (DNA polymerase) cannot create the initial DNA strand (track) because there is a part of the track right under the train. This leads to the gradual loss of important base sequences, including genes, in every cell division. Therefore, the repetitive, non-coding base sequences of telomeres at the ends of chromosomes act as a buffer to protect the essential information in cell function. Telomeres are expected to shorten progressively by about 30-200 base pairs in each cell division cycle. The length of telomeres is crucial in determining a cell’s ability to divide. As telomeres shorten with each cell division, when they reach a certain length, the cell will stop dividing permanently (senescence) or trigger self-destruction (apoptosis). This phenomenon is known as the Hayflick Limit. Telomerase is an enzyme that addresses this limitation by adding length to the telomeres. In normal conditions, telomerase functions in the early stages of embryonic development and in adult stem cells. However, it can also be active in abnormal conditions, such as in cancer cells, allowing them to proliferate extensively. In normal cells, telomerase is produced in very small quantities or not at all.
Oxygen free radicals such as O–2, H2O2, and Nitric oxide (NO) are generated from the body’s metabolic processes, relying on oxygen and occurring in the mitochondria, the organelles responsible for generating energy for cells. Some free radicals are beneficial for destroying pathogens and foreign substances. However, free radicals can also bind to the chemical bonds of DNA, proteins, and fats within cells, stealing electrons from these molecules to stabilize themselves. This causes the molecules that lose electrons to become unstable in turn, leading to a continuous chain reaction of electron theft, which can damage various substances in the cell and cause cell damage. This condition is called oxidative stress. Normally, cells contain antioxidants that provide electrons to free radicals without losing their own stability, which is a crucial factor in stopping the chain reaction. The imbalance between the amount of free radicals and antioxidants is important for the occurrence of oxidative stress. et.al. 2002)
Free radicals are a significant factor affecting telomere length. Studies have found that cells with high levels of free radicals exhibit a faster rate of telomere shortening compared to normal cells, which may indicate inefficient antioxidant activity. This telomere shortening occurs at a faster rate than the shortening caused by the “End of replication” problem. The GGG sequence of the telomeres is sensitive to oxidative stress, alkylation, or UV radiation, which can damage the DNA structure. Under oxidative stress conditions, there is an increased frequency of double-strand breaks in the telomeres. Additionally, chronic oxidative stress is linked to inflammatory diseases, and individuals with chronic conditions such as coronary artery disease, type 2 diabetes, and chronic obstructive pulmonary disease generally have shorter telomeres compared to healthy individuals. (de Vos-Houben J. M. et.al. 2002, von Zglinicki, T. 2002)
Based on research reports, oxidative stress and chronic inflammation lead to the shortening of telomeres. It is known that micronutrients such as antioxidants, vitamins, and minerals can inhibit oxidative stress and chronic inflammation. Therefore, receiving these nutrients could help delay the shortening of telomeres. Multivitamins, which contain a variety of vitamins and minerals, are a good source of micronutrients for consumption. Research studies on the consumption of multivitamins and their effects on telomere length in a female population found a correlation with longer telomeres in the group that received multivitamins compared to the control group. Nutrients and antioxidants reported to be associated with telomeres include folate, vitamin B12, vitamin A, vitamin D, nicotinamide, vitamins C and E, and omega-3. Consuming these nutrients sufficiently can help maintain the length of telomeres.
It is currently known that the shortening of telomeres is associated with cellular aging. At birth, the length of telomeres is approximately 10,000 base pairs (bp) and gradually shortens as age increases. It has been found that the average length of telomeres in white blood cells is associated with diseases related to aging, such as atherosclerosis, myocardial infarction, Alzheimer’s disease, high blood pressure, and the risk of diabetes. For example, in the group of people over 100 years old (centenarians), the average length of telomeres is found to be longer than expected. In healthy groups, the average length of telomeres is longer than in groups with various age-related diseases. Additionally, individuals with shorter average telomere length in white blood cells have an increased risk of cancer, and the mortality rate from cancer is significantly higher in individuals with shorter telomeres compared to those with longer telomeres. Several lifestyle factors also contribute to the shortening of telomeres, leading to the study of biological age, which may be important in indicating individual health risks. Previously, this was based on the relationship with chronological age, but the length of telomeres, which is both directly and indirectly related to lifestyle behaviors and health conditions, could potentially serve as a biological marker (biomarker) to assess health risks. et.al. 2015, Lin J. et.al. 2012, Sanders J.L. et.al. Therefore, to monitor changes in telomere length, it is recommended to have an annual check-up to observe the rate of telomere shortening. This can guide appropriate treatment to slow down and prevent the shortening of telomeres effectively, such as modifying sleep habits, exercising, managing stress levels, and consuming various nutrients.
Reference documents
Blackburn, E. H. “Telomeres and Telomerase: The Means to the End (Nobel Lecture).” Angew Chem Int Ed Engl 49, no. 41 (Oct 04 2010): 7405-21.
de Vos-Houben, J. M., N. R. Ottenheim, A. Kafatos, B. Buijsse, G. J. Hageman, D. Kromhout, and E. J. Giltay. “Telomere Length, Oxidative Stress, and Antioxidant Status in Elderly Men in Zutphen and Crete.” Mech Ageing Dev 133, no. 6 (Jun 2012): 373-7.
Lapham, K., M. N. Kvale, J. Lin, S. Connell, L. A. Croen, B. P. Dispensa, L. Fang, et al. “Automated Assay of Telomere Length Measurement and Informatics for 100,000 Subjects in the Genetic Epidemiology Research on Adult Health and Aging (Gera) Cohort.” Genetics 200, no. 4 (Aug 2015): 1061-72.
Lin, J., E. Epel, and E. Blackburn. “Telomeres and Lifestyle Factors: Roles in Cellular Aging.” Mutat Res 730, no. 1-2 (Feb 01 2012): 85-9.
